Research areas
Research in the Professor Sobota group has been focused around alkoxide and organometallic chemistry, and from this core area branches into catalysis, materials chemistry and organic synthesis. The group is involved with following projects:
Synthesis, structure and reactivity of organometallic and metal complexes – precursors for olefin transformations (polymerization, metathesis, Heck and Suzuki coupling, cyclization, etc.) leading to „fine chemicals” and „chemical specialities”.
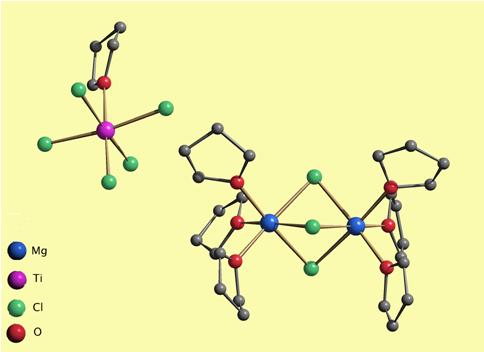
The [Mg2(µ-Cl)3(thf)6][TiCl5(thf)] compound supported on SiO2 together with an organometallic cocatalyst is used as a commercial ethylene polymerization catalyst.
Journal of Chemical Society, Dalton Transactions 1984, 2077
Polyolefin catalysts typically are formed from a precursor containing magnesium and titanium chlorides or alkoxides. For the catalyst studied, the precursor is Mg3Ti(OR)8X2 (R = alkyl, X = o-cresol or OCH2CH3). But the exact structure of the active catalyst is unknown. We instead created an isolable manganese mimic [Mn3Ti(μ3-OCH2CH2OCH3)2(μ-OCH2CH2OCH3)3(μ-Cl)Cl2(OiPr)2] containing a Mn3Ti unit and obtained its crystal structure. This model permitted us to visualize the polymerization process. We suggest that the metal atoms of the catalytic site, in which the titanium atom occupies a chiral position, impose a chiral orientation to the growing polymer chain. This effect guides the head-to-tail insertion of olefin units into the chain, which results in a stereoregular polymer.
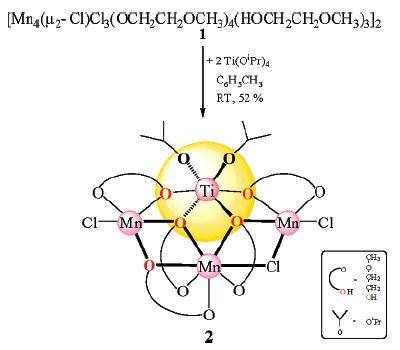
Inorganic Chemistry 2007, 46, 9024 (Scheme)
Highlighted in Chemical & Engineering News (Model Devised for Polyolefin Catalysts).
See also:
Dalton Transactions 2009, 8846
Dalton Transactions 2007, 2065
Organometallics 2005, 24, 3987
The study outlines our initial results that contribute to a better understanding of MAO/MgCl2 (MAO = methylaluminoxane) incorporation in the Cp2ZrCl2/MAO/MgCl2 catalytic system, which is currently of global industrial use. We show here that the [Al3(µ3-O)(Me)5]2+ moiety can be trapped by the tetrapodal [Mg3Cl4(thhfo)4(THF)]2- macrounit to form a cluster [Al3Mg3(µ3-O)(thffo)4(Me)5Cl4(THF)] (thffo = 2-tetrahydrofurfuroxide). From this perspective, this macrounit might be considered as a part of the MgCl2 support surface, which fulfills the requirement of a Al3(μ3-O) core.
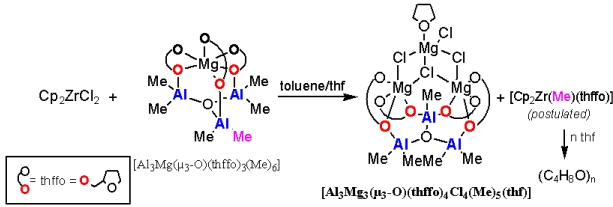
Inorganic Chemistry 2005, 44, 9131 (Scheme)
See also:
European Journal of Inorganic Chemistry 2005, 1063
Synthesis, structure and thermal decomposition of single-source precursors for mono- and double oxides with perovskite and spinel-like structures.
Potential single-source molecular precursors [Ba{(μ-ddbfo)2MRx}2] (ddbfoH) 2,3-dihydro-2,2-dimethylbenzofuran-7-ol; M/R/x ) Al/Me/2, Al/Et/2, Ga/Me/2, or Zn/Et/1) for mixed metal oxide materials were prepared by the reaction of barium aryloxide [Ba(ddbfo)2(ddbfoH)2]·3ddbfoH with an appropriate organometallic compound in toluene. The precursors were characterized by elemental analysis, IR and NMR spectroscopy, and single-crystal X-ray structural analysis. The Ba/Al2 and Ba/Ga2 complexes undergo thermal decomposition to give mixed metal oxides BaM2O4 (M = Al, at 1300 °C for both Me and Et derivatives; Ga, at 1430 °C). Instead, the Ba/Zn2 complex decomposes to a mixture of BaCO3 and different metal oxides. The chemical composition and surface morphology of heterobimetallic oxides were analyzed by SEM-EDS and X-ray powder diffraction (XRD) that revealed the formation of highly pure oxide materials with micrometer particle sizes.
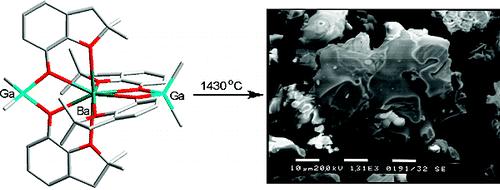
Chemistry of Materials 2008, 20, 4231 (Scheme)
See also:
Inorganic Chemistry 2009, 48, 6584
Inorganic Chemistry 2008, 47, 7939
Synthesis of metal complexes and organometallic compounds-precursors for electronic and magnetic materials as well as biomaterials.
A simple and unique route to access the heterometallic cluster [Mn4Ti4(µ-Cl)2(µ3,η2-L)2(µ,η2-L)10Cl6] (1) with two Mn2Ti2 butterfly core motifs is reported. This method comprises elimination of the Cp ring from Cp2TiCl2 as CpH in the presence of metallic Mn in 2-methoxoethanol (LH) as a proton source. Complex 1 belongs to a group of magnetic clusters, which consists of two weakly interacting M4 subunits.
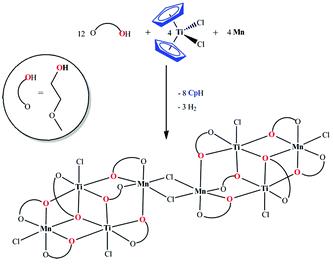
Dalton Transactions 2009, 5450 (Scheme)
See also:
Dalton Transactions (Perspective) 2008, 6509
New catalysts for biodegradable and environment friendly polymers.
The reaction of ZnEt2 with one or two equivalents of aminophenolate ligand N-[methyl(2-hydroxy-3,5-di-tert-butylphenyl)]-N-methyl-N-cyclohexylamine gives hetero- and homoleptic molecular compounds [(µ,η2-L2)ZnEt]2 and [Zn(η2-L2)2]. The later is most probably a mixture of diastereoisomers that in solution shows an interesting dynamic behaviour. Both complexes as well as the BnOH derivative of the latter, [(η2-L2)Zn(µ-BnO)]2, proved excellent initiators for lactide polymerization.
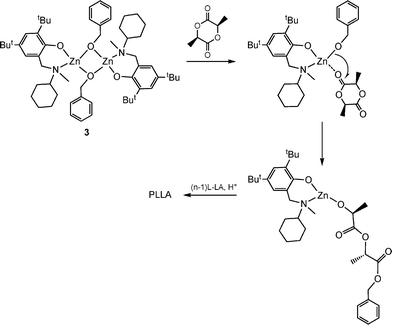
Dalton Transactions 2008, 6556 (Scheme)
See also:
Macromolecules 2009, 42, 4008
Journal of Molecular Catalysis 2006, 257, 105
Inorganic Chemistry 2006, 45, 5302
Dalton Transactions 2005, 2047
Chemical models of the active site of nitrogenase and manganese-calcium clusters relevant to photosynthetic dioxygen-evolving centers.
We describe here a novel, simple, efficient self-assembly method for the in situ generation of [Mn4Cl4(µ-OCH2CH2OMe)4(EtOH)4] (1) and [Mn4(µ-Cl)Cl3(µ-OCH2CH2OMe)4(HOCH2CH2OMe)3]2 (2) cubane-type compounds which react readily with calcium species to form cluster [Mn4Ca2Cl4(µ-OCH2CH2OMe)8] (3), the calcium atoms attached to the Mn4 unit of 3 flatten out the cubane inducing significant conformational changes.
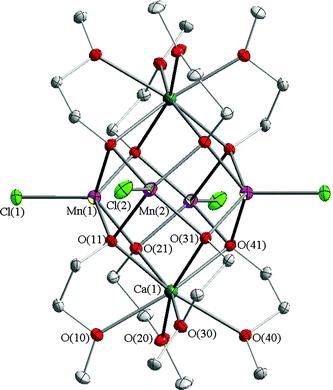
Dalton Transactions 2007, 825 (Figure)
Synthesis of organic and organometallic polyynes with potential use as nanoscale electronic devices.
The reactions of precursors 4-BrC6H4COR with TMSC≡CH (Sonogashira coupling) followed by in situ deprotection and subsequent dimerization gave thermally stable dimeric keto-diynes RCOC6H4(C≡C)2C6H4COR (8-11) as yellow powders in 25-85 % yields without the necessity of carbonyl group protection/deprotection steps. Compounds 8-11 were also synthesized by an alternative method that utilized α-haloalkynes previously obtained directly from TMS-protected 4-RCOC6H4C≡CTMS alkynes. The resulting diynes were characterized by spectroscopic methods and in most cases by X-ray crystallography. Careful analysis of the crystal data revealed a surprisingly high degree of chain curvature. Moreover, compounds 9 (R = Me) and 10 (R = Et) were extremely flat, which greatly facilitates electronic communication throughout the whole molecule. Deeper analysis of the packing motifs showed 9 to have a great potential for topochemical 1,4-polymerization.
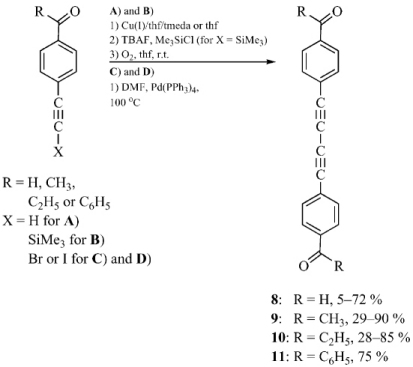
European Journal of Organic Chemistry 2008, 27, 4598 (Scheme)